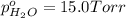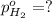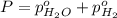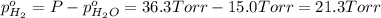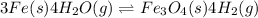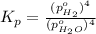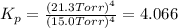Chemistry, 12.12.2019 04:31 makalaily9342
In a study of the following reaction at 1200 k it was observed that when the equilibrium partial pressure of water vapor is 15.0 torr, the total pressure at equilibrium is 36.3 torr. 3 fe(s) 4 h2o(g) equilibrium reaction arrow fe3o4(s) 4 h2(g) calculate the value of kp for this reaction at 1200 k. hint: apply dalton's law of partial pressures.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
In a study of the following reaction at 1200 k it was observed that when the equilibrium partial pre...
Questions

Mathematics, 23.02.2021 21:40



English, 23.02.2021 21:40

Chemistry, 23.02.2021 21:40

Chemistry, 23.02.2021 21:40

Mathematics, 23.02.2021 21:40




Physics, 23.02.2021 21:40

Social Studies, 23.02.2021 21:40




Mathematics, 23.02.2021 21:40

Computers and Technology, 23.02.2021 21:40



Biology, 23.02.2021 21:40

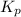 for this reaction at 1200 K is 4.066.
for this reaction at 1200 K is 4.066.