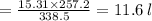Chemistry, 12.12.2019 05:31 laykaleb086
Asample of ammonia gas at 65.5°c and 524 torr has a volume of 15.31 l. what is its volume when the temperature is –15.8°c and its pressure is 524 torr? 20.2 l 11.6 l 63.5 l not possible, since the volume would have to be negative 3.69 l

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1


Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
You know the right answer?
Asample of ammonia gas at 65.5°c and 524 torr has a volume of 15.31 l. what is its volume when the t...
Questions


Mathematics, 05.09.2021 08:50

English, 05.09.2021 08:50

English, 05.09.2021 08:50

Mathematics, 05.09.2021 08:50

English, 05.09.2021 08:50

Mathematics, 05.09.2021 08:50

Mathematics, 05.09.2021 08:50

English, 05.09.2021 08:50

English, 05.09.2021 08:50

Advanced Placement (AP), 05.09.2021 08:50

Business, 05.09.2021 08:50

Mathematics, 05.09.2021 08:50



Physics, 05.09.2021 09:00

Mathematics, 05.09.2021 09:00

History, 05.09.2021 09:00

Business, 05.09.2021 09:00

Mathematics, 05.09.2021 09:00