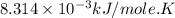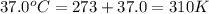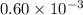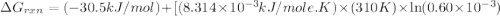Chemistry, 12.12.2019 05:31 eileentennyson
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by the reaction atp ( aq ) + h 2 o ( l ) ⟶ adp ( aq ) + hpo 2 − 4 ( aq ) for which δ g ∘ rxn = − 30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δ g rxn in a biological cell in which [ atp ] = 5.0 mm, [ adp ] = 0.60 mm, and [ hpo 2 − 4 ] = 5.0 mm.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions

History, 17.09.2019 16:00


Mathematics, 17.09.2019 16:00


Mathematics, 17.09.2019 16:00




Mathematics, 17.09.2019 16:00

Biology, 17.09.2019 16:00

Social Studies, 17.09.2019 16:00

English, 17.09.2019 16:00

History, 17.09.2019 16:00


English, 17.09.2019 16:00


Mathematics, 17.09.2019 16:00

Chemistry, 17.09.2019 16:00


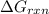 is -49.6 kJ/mol
is -49.6 kJ/mol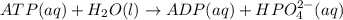
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0414/8690/ccdf0.png)
![[ATP]](/tpl/images/0414/8690/bda18.png) = 5.0 mM
= 5.0 mM![[ADP]](/tpl/images/0414/8690/68360.png) = 0.60 mM
= 0.60 mM![[HPO_4^{2-}]](/tpl/images/0414/8690/c0ca9.png) = 5.0 mM
= 5.0 mM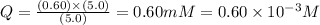
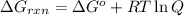 ............(1)
............(1)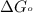 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol