
Chemistry, 12.12.2019 05:31 david1236544
Three identical flasks contain three different gases at standard temperature and pressure. flask a contains flask b contains o3, and flask c contains n2. which flask contains the largest number of molecules? a) flask a b) flask b c) flask c d) all contain same number of molecules.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
Three identical flasks contain three different gases at standard temperature and pressure. flask a c...
Questions


Health, 28.09.2019 13:30

History, 28.09.2019 13:30

Health, 28.09.2019 13:30

Business, 28.09.2019 13:30

Business, 28.09.2019 13:30




Mathematics, 28.09.2019 13:30



Mathematics, 28.09.2019 13:30

Mathematics, 28.09.2019 13:30




English, 28.09.2019 13:30


Chemistry, 28.09.2019 13:30

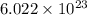 number of molecules
number of molecules

