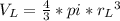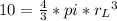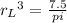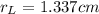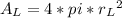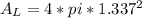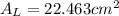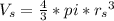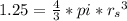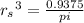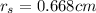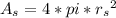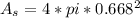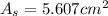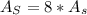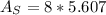Chemistry, 12.12.2019 05:31 williamslyric
In a certain industrial process involving a heterogeneous catalyst, the volume of the catalyst (in the shape of a sphere) is 10.0 cm3. if the sphere were broken down into eight spheres each having a volume of 1.25 cm3, and the reaction is run a second time, which of the following accurately characterizes the second run? choose all that apply a. the second run will be faster. b. the second run will be slower. c. the second run will have the same rate as the first. d. the second run has twice the surface area. e.the second run has eight times the surface area. f. the second run has 10 times the surface area

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow.part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer.o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part.h2o is a stronger acid than oh–, so the equilibrium lies to the right.h2o is a weaker acid than oh–, so the equilibrium lies to the left.h2o is a stronger acid than oh–, so the equilibrium lies to the left.h2o is a weaker acid than oh–, so the equilibrium lies to the right.part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer.ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-(aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left.part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer.no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part.hno2 is a stronger acid than h2o, so the equilibrium lies to the right.hno2 is a weaker acid than h2o, so the equilibrium lies to the left.hno2 is a stronger acid than h2o, so the equilibrium lies to the left.hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
In a certain industrial process involving a heterogeneous catalyst, the volume of the catalyst (in t...
Questions

Mathematics, 27.05.2021 22:50

Biology, 27.05.2021 22:50



Mathematics, 27.05.2021 22:50

Mathematics, 27.05.2021 22:50

Mathematics, 27.05.2021 22:50




Mathematics, 27.05.2021 22:50

Social Studies, 27.05.2021 22:50


History, 27.05.2021 22:50

Mathematics, 27.05.2021 22:50

Mathematics, 27.05.2021 22:50

Computers and Technology, 27.05.2021 22:50


Engineering, 27.05.2021 22:50