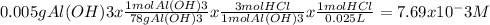Chemistry, 12.12.2019 05:31 nathanscastr02
Antacids are compounds, usually bases, used to decrease the amount of hydrochloric acid in the stomach. write a brief titration procedure for determining how many moles of acid a single antacid tablet would neutralize. this procedure should be detailed and correct so that someone could go into a laboratory and follow the procedure you have written.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 13:30
The process by which liquid water changes into water vapour is called what ?
Answers: 2

Chemistry, 23.06.2019 15:30
Can someone me with these problems? see the attachment, .
Answers: 1
You know the right answer?
Antacids are compounds, usually bases, used to decrease the amount of hydrochloric acid in the stoma...
Questions

Biology, 09.12.2021 01:40

Physics, 09.12.2021 01:40



History, 09.12.2021 01:40


Mathematics, 09.12.2021 01:40

French, 09.12.2021 01:40

Mathematics, 09.12.2021 01:40

French, 09.12.2021 01:40

Biology, 09.12.2021 01:40

Biology, 09.12.2021 01:40



SAT, 09.12.2021 01:40


Mathematics, 09.12.2021 01:40