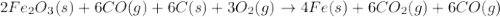Blast furnaces extract pure iron from the iron(iii) oxide in iron ore in a two step sequence. in the first step, carbon and oxygen react to form carbon monoxide: 2c (s) + o2 (g) → 2co (g)in the second step, iron(iii) oxide and carbon monoxide react to form iron and carbon dioxide: fe2o3 (s) + 3co (g) → 2fe (s) + 3co2 (g)write the net chemical equation for the production of iron from carbon, oxygen and iron(iii) oxide. be sure your equation is balanced.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Blast furnaces extract pure iron from the iron(iii) oxide in iron ore in a two step sequence. in the...
Questions

Mathematics, 25.01.2021 17:40

Advanced Placement (AP), 25.01.2021 17:40

Advanced Placement (AP), 25.01.2021 17:40



Social Studies, 25.01.2021 17:40


Social Studies, 25.01.2021 17:40

Social Studies, 25.01.2021 17:40

Mathematics, 25.01.2021 17:40



Mathematics, 25.01.2021 17:40


English, 25.01.2021 17:40

Mathematics, 25.01.2021 17:40

Mathematics, 25.01.2021 17:40

Social Studies, 25.01.2021 17:40

Mathematics, 25.01.2021 17:40

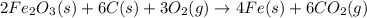
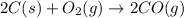 ..[1]
..[1]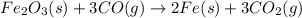 ..[2]
..[2]