Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
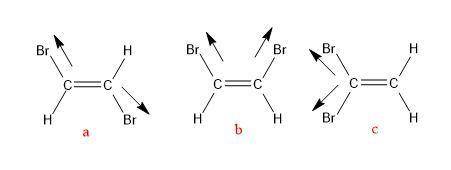
There are three different possible isomers of a dibromoethene molecule, c2h2br2c2h2br2 . one of them...
Questions


Mathematics, 31.10.2019 00:31

English, 31.10.2019 00:31

Physics, 31.10.2019 00:31

Social Studies, 31.10.2019 00:31




Mathematics, 31.10.2019 00:31

Biology, 31.10.2019 00:31

Mathematics, 31.10.2019 00:31

Chemistry, 31.10.2019 00:31

History, 31.10.2019 01:31



Mathematics, 31.10.2019 01:31


History, 31.10.2019 01:31

Mathematics, 31.10.2019 01:31

Mathematics, 31.10.2019 01:31