Chemistry, 13.12.2019 00:31 JohnJamesPaksitani
Hydrogen peroxide may decompose to form water and oxygen gas according to the following reaction 2h202(g) 2h20(g)+02(g) in a particular experiment, 1.75 moles of hy02 were placed in a 25-l reaction chamber at 307ec after equilibrium was reached, 1.20 moles of h202 remained what is ke for the reaction?
a. 2.4×10^-3
b. 2.0×10^-4
c. 5.5×10^-3
d. 2.3×10^-2

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1
You know the right answer?
Hydrogen peroxide may decompose to form water and oxygen gas according to the following reaction 2h2...
Questions


Computers and Technology, 05.05.2020 05:25


Physics, 05.05.2020 05:25

Mathematics, 05.05.2020 05:25

Mathematics, 05.05.2020 05:25













Mathematics, 05.05.2020 05:25

English, 05.05.2020 05:25

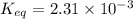
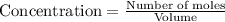
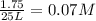
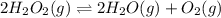
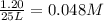
![[H_2O]=2x=2\times 0.011 M= 0.022 M](/tpl/images/0416/0677/428c7.png)
![[O_2]=x=0.011 M](/tpl/images/0416/0677/5d441.png)
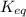 for the above reaction follows:
for the above reaction follows:![K_{eq}=\frac{[H_2O]^2\times [O_2]}{ [H_2O_2]^2}](/tpl/images/0416/0677/4f6fe.png)