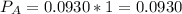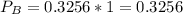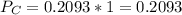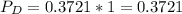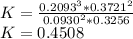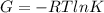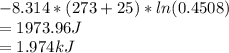Chemistry, 13.12.2019 01:31 Bryson2148
In the gas-phase reaction 2 a + b ⇌ 3 c + 2 d, it was found that, when 1.00 mol a, 2.00 mol b, and 1.00 mol d were mixed and allowed to come to equilibrium at 25 °c, the resulting mixture contained 0.90 mol c at a total pressure of 1.00 bar. calculate
(i) the mole fractions of each species at equilibrium,
(ii) k, and
(iii) δrimage.

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2


Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3
You know the right answer?
In the gas-phase reaction 2 a + b ⇌ 3 c + 2 d, it was found that, when 1.00 mol a, 2.00 mol b, and 1...
Questions

Health, 20.10.2020 23:01


Mathematics, 20.10.2020 23:01






History, 20.10.2020 23:01

English, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01





Mathematics, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01


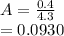
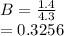
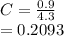
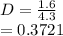
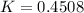
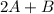 ⇄
⇄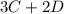
 be the number of moles dissociated per mole of
be the number of moles dissociated per mole of 
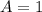
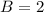
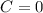
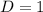
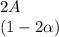 +
+ 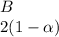 ⇄
⇄ 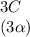 +
+ 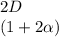
![C[tex] is 0.9Thus ,[tex]3\alpha=0.9\\\alpha=0.3[tex]The final number of moles of:[tex]A = 1-2\alpha=1-2*0.3=0.4mol[tex] [tex]B=2(1-\alpha)=2(1-0.3)=1.4mol[tex][tex]D=1+2\alpha=1+2*0.3=1.6mol[tex]Thus , total number of moles are : 0.4+1.4+0.9+1.6=4.3(i)The mole fractions are : [tex]A=\frac{0.4}{4.3} \\=0.0930](/tpl/images/0416/2107/4a2fd.png)
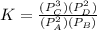
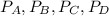 are the partial pressures of A,B,C,D respectively.
are the partial pressures of A,B,C,D respectively.