Consider the following chemical equation. how many moles of seo3 can be produced from 35
g of...

Chemistry, 13.12.2019 01:31 karinagramirezp072gb
Consider the following chemical equation. how many moles of seo3 can be produced from 35
g of oxygen and excess selenium?
25e +302 - 25e03

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
Questions


Mathematics, 09.11.2020 20:30

Mathematics, 09.11.2020 20:30

Business, 09.11.2020 20:30

Mathematics, 09.11.2020 20:30



Mathematics, 09.11.2020 20:30

Mathematics, 09.11.2020 20:30




Mathematics, 09.11.2020 20:40

Physics, 09.11.2020 20:40

Mathematics, 09.11.2020 20:40

English, 09.11.2020 20:40


Medicine, 09.11.2020 20:40


Mathematics, 09.11.2020 20:40

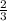 moles of SeO3
moles of SeO3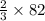 g of SeO3
g of SeO3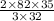 g of SeO3
g of SeO3 

