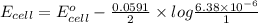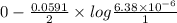Chemistry, 13.12.2019 02:31 tmgoddess2004
The ksp of copper(ii) ferrocyanide (cu2[fe(cn)6]) is 1.3 × 10−16 at 25°c. determine the potential of a concentration cell in which one half-cell consists of a copper electrode in 1.00 m copper(ii) nitrate, and the other consists of a copper electrode in a saturated solution of cu2[fe(cn)6].
ferrocyanide, ([fe(cn)6]4−), is a complex ion.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
The ksp of copper(ii) ferrocyanide (cu2[fe(cn)6]) is 1.3 × 10−16 at 25°c. determine the potential of...
Questions

History, 20.02.2020 09:14

Mathematics, 20.02.2020 09:15

Mathematics, 20.02.2020 09:15



English, 20.02.2020 09:15



Social Studies, 20.02.2020 09:15



Social Studies, 20.02.2020 09:15




Mathematics, 20.02.2020 09:16


Geography, 20.02.2020 09:16


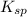 of the given reaction is as follows.
of the given reaction is as follows.![K_{sp} = [Cu^{2+}]^{2}[Fe(CN)_{6}]](/tpl/images/0416/3120/586ea.png)
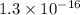 .
.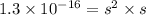
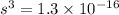
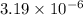
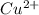 will be calculated as follows.
will be calculated as follows.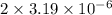
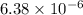 M
M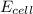 as follows.
as follows.