
Chemistry, 13.12.2019 03:31 carlosleblanc26
After distilling your crude methyl benzoate, you set aside 5.12 grams of the purified ester. you then prepare the grignard reagent ( phenylmagnesium bromide ) by reacting 2.3 grams of magnesium with 9.45 ml of bromobenzene. you add the 5.12 grams of methyl benzoate to the freshly prepared grignard reagent to form an addition product. finally, after hydrolyzing the grignard addition product, you obtain 5.3 grams of the final product, triphenyl carbinol. what is the percent yield of triphenyl carbinol ? ( the density of bromobenzene is 1.495 g/ml, triphenyl carbinol = 260.33 g/mol, bromobenzene = 157.01 g/mol, mg = 24.3 g/mol )

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1
You know the right answer?
After distilling your crude methyl benzoate, you set aside 5.12 grams of the purified ester. you the...
Questions

Mathematics, 03.11.2020 16:20

English, 03.11.2020 16:20




Biology, 03.11.2020 16:20


Mathematics, 03.11.2020 16:20

Mathematics, 03.11.2020 16:20



Mathematics, 03.11.2020 16:20








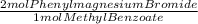 = 0.0752 moles of phenylmagnesium bromide. As you have 0.900 moles of phenylmagnesium bromide, limiting reagent is Methyl benzoate and moles of triphenyl carbinol are 0.0376. In grams:
= 0.0752 moles of phenylmagnesium bromide. As you have 0.900 moles of phenylmagnesium bromide, limiting reagent is Methyl benzoate and moles of triphenyl carbinol are 0.0376. In grams:

