
Chemistry, 13.12.2019 03:31 bentonknalige
Suppose 0.245 g of sodium chloride is dissolved in 50. ml of a 18.0 m m aqueous solution of silver nitrate.
calculate the final molarity of chloride anion in the solution. you can assume the volume of the solution doesn’t change when the sodium chloride is dissolved in it.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Suppose 0.245 g of sodium chloride is dissolved in 50. ml of a 18.0 m m aqueous solution of silver n...
Questions

Geography, 25.04.2021 05:50

Mathematics, 25.04.2021 05:50

Mathematics, 25.04.2021 05:50


Mathematics, 25.04.2021 05:50


World Languages, 25.04.2021 05:50


English, 25.04.2021 06:00


Mathematics, 25.04.2021 06:00

Mathematics, 25.04.2021 06:00

Mathematics, 25.04.2021 06:00

World Languages, 25.04.2021 06:00

Mathematics, 25.04.2021 06:00

Mathematics, 25.04.2021 06:00

Mathematics, 25.04.2021 06:00

Mathematics, 25.04.2021 06:00

Physics, 25.04.2021 06:00

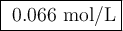
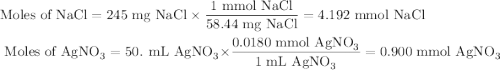
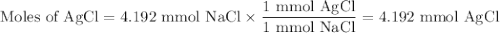
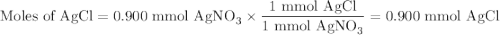
![\text{[Cl$^{-}$] } = \dfrac{\text{3.292 mmol}}{\text{50. mL}} = \textbf{0.066 mol/L}\\\text{The concentration of chloride ion is $\large \boxed{\textbf{0.066 mol/L}}$}](/tpl/images/0416/3996/2071d.png)


