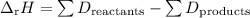Chemistry, 13.12.2019 03:31 mikayla843
Calculate the average bond energy of the sulfur-oxygen bonds in so2 (in kj/mol), given the following information. sf4(g) + 2 h2o(g) → so2(g) + 4 hf(g) δh = –123 kj bond dissociation energies: s–f 327 kj/mol f–f 154 kj/mol h–f 565 kj/mol h–o 467 kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
Calculate the average bond energy of the sulfur-oxygen bonds in so2 (in kj/mol), given the following...
Questions

Computers and Technology, 15.07.2019 20:30




World Languages, 15.07.2019 20:30



Biology, 15.07.2019 20:30


Biology, 15.07.2019 20:30

Mathematics, 15.07.2019 20:30




Mathematics, 15.07.2019 20:30




Biology, 15.07.2019 20:30

Social Studies, 15.07.2019 20:30