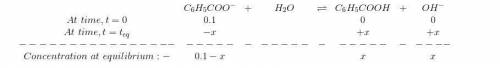Sodium benzoate (c6h5coona), the sodium salt of the weak acid benzoic acid, is used as a food preservative. a solution is prepared by dissolving 0.100 mol of sodium benzoate in enough pure water to produce 1.00 l of solution. if the pka for benzoic acid is 4.20, calculate the ph of the sodium benzoate solution.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:10
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1

Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1

You know the right answer?
Sodium benzoate (c6h5coona), the sodium salt of the weak acid benzoic acid, is used as a food preser...
Questions

History, 22.11.2020 07:50

Mathematics, 22.11.2020 07:50

Mathematics, 22.11.2020 07:50

Mathematics, 22.11.2020 07:50

Mathematics, 22.11.2020 07:50

History, 22.11.2020 07:50


English, 22.11.2020 07:50

Mathematics, 22.11.2020 07:50

French, 22.11.2020 07:50

Mathematics, 22.11.2020 07:50


Computers and Technology, 22.11.2020 07:50

Social Studies, 22.11.2020 07:50

History, 22.11.2020 07:50

English, 22.11.2020 07:50


Mathematics, 22.11.2020 07:50

Social Studies, 22.11.2020 07:50

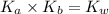
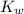 is the dissociation constant of water.
is the dissociation constant of water. ,
, 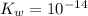
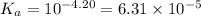
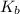 for Sodium benzoate can be calculated as:
for Sodium benzoate can be calculated as: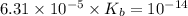
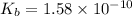
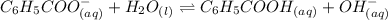
![K_{b}=\frac {\left [ C_6H_5COOH^{+} \right ]\left [ {OH}^- \right ]}{[C_6H_5COO^-]}](/tpl/images/0416/3772/af673.png)
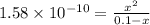

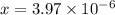
![[OH^-]=3.97\times 10^{-6}](/tpl/images/0416/3772/71435.png)
![pOH=-log[OH^-]=-log(3.97\times 10^{-6})=5.4](/tpl/images/0416/3772/9fc03.png)