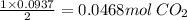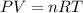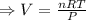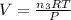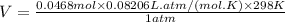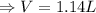Chemistry, 13.12.2019 04:31 redbeast677
The following is the balanced equation of the combustion of methane gas with oxygen gas:
ch4(g) + 2o2(g) -> co2(g) + 2h2o(l)
if you ran a reaction in a sealed balloon at 25o c with 1 g of methane, ch4, and 3 g of oxygen, o2, what is the end volume of the balloon once the reaction is complete? the pressure was constant at 1 atm. assume all gases behave ideally and you have 100% yield. (ignore vapor pressure of water)
a. 4.9 l
b. 0.096 l
c. 1.1 l
d 1.5 l
e. 0.13 l

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
The following is the balanced equation of the combustion of methane gas with oxygen gas:
Questions

Mathematics, 08.04.2020 01:36

Social Studies, 08.04.2020 01:36







Mathematics, 08.04.2020 01:36


Mathematics, 08.04.2020 01:36

History, 08.04.2020 01:36

Biology, 08.04.2020 01:36





Mathematics, 08.04.2020 01:36


Biology, 08.04.2020 01:37

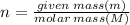
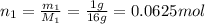
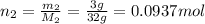
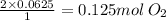 .
.