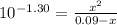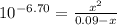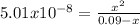Phosphorous acid, h3po3(aq), is a diprotic oxyacid that is an important compound in industry and agriculture. k pka1 k pka2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.8 m h3po3(aq) with 1.8 m koh(aq). before addition of any koh: after addition of 25.0 ml koh: after addition of 50.0 ml koh: after addition of 75.0 ml koh: after addition of 100.0 ml koh:

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
Phosphorous acid, h3po3(aq), is a diprotic oxyacid that is an important compound in industry and agr...
Questions

Mathematics, 05.05.2020 12:28



Chemistry, 05.05.2020 12:28


Chemistry, 05.05.2020 12:28

Mathematics, 05.05.2020 12:28

Mathematics, 05.05.2020 12:28

History, 05.05.2020 12:28

Social Studies, 05.05.2020 12:28

Mathematics, 05.05.2020 12:28

Mathematics, 05.05.2020 12:28

Mathematics, 05.05.2020 12:28


History, 05.05.2020 12:28

Mathematics, 05.05.2020 12:28

Mathematics, 05.05.2020 12:28



Mathematics, 05.05.2020 12:29