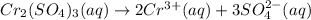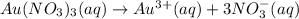Chemistry, 13.12.2019 06:31 robertss403
Iwant to use a galvanic cell to power a 60-watt light bulb. complete the following steps to determine how long the galvanic cell will power the light bulb before running out.
a.) the galvanic cell uses the following solutions: 0.81 l 1.5 m cr2(so4)3 solution and 1.2 l 0.81 m au(no3)3 solution. write down the balanced chemical equations of the dissolution of cr2(so4)3and au(no3)3 into ions in water.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1
You know the right answer?
Iwant to use a galvanic cell to power a 60-watt light bulb. complete the following steps to determin...
Questions

Mathematics, 05.02.2021 18:30






Chemistry, 05.02.2021 18:30

Mathematics, 05.02.2021 18:30


Spanish, 05.02.2021 18:30

Mathematics, 05.02.2021 18:30


French, 05.02.2021 18:30


English, 05.02.2021 18:30


Mathematics, 05.02.2021 18:30


Mathematics, 05.02.2021 18:30

Mathematics, 05.02.2021 18:30