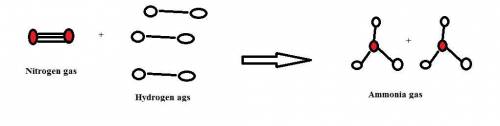
Chemistry, 13.12.2019 06:31 antonjas001
1. how many molecules of ammonia can be created when four molecules of nitrogen are combined with four molecules of hydrogen? include a drawing of the molecules in your answer.
2. what ratio of nitrogen and hydrogen molecules would result in no left-over reactants? explain your answer
3. if 100.0g of nitrogen is reacted with 100.0g of hydrogen, what is the theoretical yield of the reaction? what is the excess reactant? what is the limiting reactant?
me i'm so confused

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
1. how many molecules of ammonia can be created when four molecules of nitrogen are combined with fo...
Questions




History, 17.09.2019 14:10

Social Studies, 17.09.2019 14:10



Physics, 17.09.2019 14:10

Mathematics, 17.09.2019 14:10


Health, 17.09.2019 14:10

Biology, 17.09.2019 14:10

Mathematics, 17.09.2019 14:10

Business, 17.09.2019 14:10




Mathematics, 17.09.2019 14:10


Mathematics, 17.09.2019 14:10

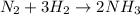
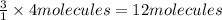 of hydrogen gas.
of hydrogen gas.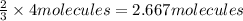 of ammonia
of ammonia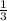 = 1 : 3
= 1 : 3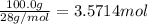
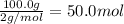
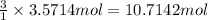 of hydrogen gas.
of hydrogen gas.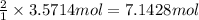 of ammonia
of ammonia