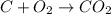Chemistry, 26.08.2019 16:30 xXFLUFFYXx
Which observation illustrates the law of conservation of mass?
the c: o mass ratio of a particular compound is the same, regardless of the size or source of the sample.
when 3 g of carbon reacts with 8 g of oxygen, 11 g of carbon dioxide is produced.
the c: o mass ratio of one compound is exactly double that of another compound.
burning 10 g of propane produces twice as much carbon dioxide as burning 5 g of propane.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
Which observation illustrates the law of conservation of mass?
the c: o mass ratio of a parti...
the c: o mass ratio of a parti...
Questions

English, 31.08.2019 15:30

Biology, 31.08.2019 15:30

History, 31.08.2019 15:30

History, 31.08.2019 15:30




Mathematics, 31.08.2019 15:30


Mathematics, 31.08.2019 15:30



Mathematics, 31.08.2019 15:30


Mathematics, 31.08.2019 15:30



Biology, 31.08.2019 15:30

Mathematics, 31.08.2019 15:30

Mathematics, 31.08.2019 15:30