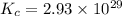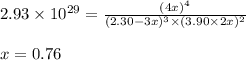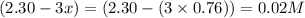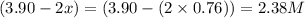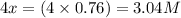Chemistry, 13.12.2019 18:31 zakarycrane9576
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium constant, kc. 3 a ( g ) + 2 b ( g ) − ⇀ ↽ − 4 c ( g ) k c = 2.93 × 10 29 if, at this temperature, 2.30 mol of a and 3.90 mol of b are placed in a 1.00 l container, what are the concentrations of a, b, and c at equilibrium?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

You know the right answer?
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium consta...
Questions



Social Studies, 25.03.2021 21:30

Mathematics, 25.03.2021 21:30

English, 25.03.2021 21:30

Mathematics, 25.03.2021 21:30



Mathematics, 25.03.2021 21:30


Mathematics, 25.03.2021 21:30

History, 25.03.2021 21:30






Mathematics, 25.03.2021 21:30


Mathematics, 25.03.2021 21:30

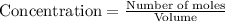
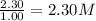
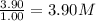
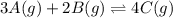
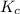 for above equation follows:
for above equation follows:![K_c=\frac{[C]^4}{[A]^3\times [B]^2}](/tpl/images/0417/2973/a92d9.png)