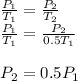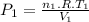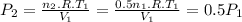Chemistry, 13.12.2019 19:31 gstinson98
Assume that you have a cylinder with a movable piston. what would happen to the gas pressure inside the cylinder if you do the following? part a: decrease the volume to one-fourth the original volume while holding the temperature constant. express your answer in terms of the variable p initial. part b: reduce the kelvin temperature to half its original value while holding the volume constant. express your answer in terms of the variable p initial .part c: reduce the amount of gas to half while keeping the volume and temperature constant. express your answer in terms of the variable p initial .

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

You know the right answer?
Assume that you have a cylinder with a movable piston. what would happen to the gas pressure inside...
Questions

Mathematics, 14.07.2019 12:00





Biology, 14.07.2019 12:00

Mathematics, 14.07.2019 12:00


Health, 14.07.2019 12:00



Mathematics, 14.07.2019 12:00


Mathematics, 14.07.2019 12:00


Mathematics, 14.07.2019 12:00

Mathematics, 14.07.2019 12:00


Mathematics, 14.07.2019 12:00

History, 14.07.2019 12:00