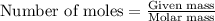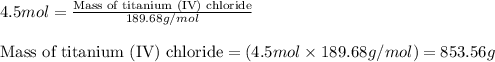Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
Titanium metal reacts with chlorine gas to form titanium (iv) chloride. what is the theoretical yiel...
Questions


Arts, 03.01.2021 15:00

Mathematics, 03.01.2021 15:00

English, 03.01.2021 15:00

Arts, 03.01.2021 15:00


Arts, 03.01.2021 15:00




Mathematics, 03.01.2021 15:00


Spanish, 03.01.2021 15:00

Mathematics, 03.01.2021 15:00

Mathematics, 03.01.2021 15:00


Computers and Technology, 03.01.2021 15:00


Mathematics, 03.01.2021 15:00

Social Studies, 03.01.2021 15:00

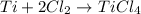
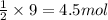 of titanium metal.
of titanium metal.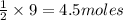 of titanium (IV) chloride.
of titanium (IV) chloride.