
Consider the following system at equilibrium where \delta h° = 18.8 kj, and kc = 9.52×10-2, at 350 k.
ch4(g) + ccl4(g) \rightleftharpoons 2ch2cl2(g)
when 0.31 moles of ccl4(g) are removed from the equilibrium system at constant temperature:
1. the value of
a. increases.
b. decreases.
c. remains the same.
2. the value of qc
a. is greater than
b. is equal to kc.
c. is less than kc.
3. the reaction :
a. run in the forward direction to reestablish equilibrium.
b. run in the reverse direction to reestablish equilibrium.
c. remain the same. it is already at equilibrium.
4. the concentration of ch4 will:
a. increase.
b. decrease.
c. remain the same.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

You know the right answer?
Consider the following system at equilibrium where \delta h° = 18.8 kj, and kc = 9.52×10-2, at 350 k...
Questions

Chemistry, 25.04.2021 02:10

History, 25.04.2021 02:10

History, 25.04.2021 02:10



Mathematics, 25.04.2021 02:10

History, 25.04.2021 02:10






Mathematics, 25.04.2021 02:10

Mathematics, 25.04.2021 02:10

Spanish, 25.04.2021 02:10


Mathematics, 25.04.2021 02:10

English, 25.04.2021 02:10


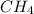 will increase because product will be converted to reactants to reestablish equilibrium.
will increase because product will be converted to reactants to reestablish equilibrium.

