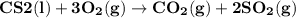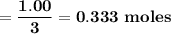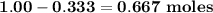Carbon disulfide burns in oxygen to yield carbon dioxide and sulfur dioxide according to the following chemical equation. cs2(l) + 3o2(g) → co2(g) + 2so2(g)
a. if 1.00 mol cs2 reacts with 1.00 mol o2, identify the limiting reactant.
b. how many moles of excess reactant remain?
c. how many moles of each product are formed?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3


Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
Carbon disulfide burns in oxygen to yield carbon dioxide and sulfur dioxide according to the followi...
Questions


History, 04.08.2019 04:40

Physics, 04.08.2019 04:40


Mathematics, 04.08.2019 04:40



Biology, 04.08.2019 04:40

Mathematics, 04.08.2019 04:40



Business, 04.08.2019 04:40

English, 04.08.2019 04:40

Mathematics, 04.08.2019 04:40

Health, 04.08.2019 04:40

Social Studies, 04.08.2019 04:40


Mathematics, 04.08.2019 04:40


History, 04.08.2019 04:40