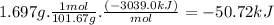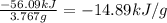Chemistry, 14.12.2019 03:31 mahmudabiazp3ekot
At constant volume, the heat of combustion of a particular compound, compound a, is − 3039.0 kj / mol. when 1.697 g of compound a (molar mass = 101.67 g / mol ) is burned in a bomb calorimeter, the temperature of the calorimeter (including its contents) rose by 3.661 ∘ c. what is the heat capacity (calorimeter constant) of the calorimeter? c = kj/°c suppose a 3.767 g sample of a second compound, compound b, is combusted in the same calorimeter, and the temperature rises from 23.23 ∘ c to 27.28 ∘ c. what is the heat of combustion per gram of compound b?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
At constant volume, the heat of combustion of a particular compound, compound a, is − 3039.0 kj / mo...
Questions

Mathematics, 18.12.2020 07:30





English, 18.12.2020 07:30




Mathematics, 18.12.2020 07:30




Social Studies, 18.12.2020 07:30




History, 18.12.2020 07:30

Mathematics, 18.12.2020 07:30

Physics, 18.12.2020 07:30