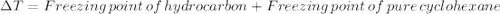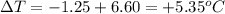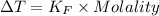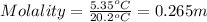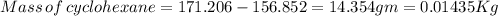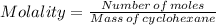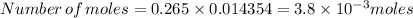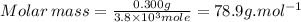Chemistry, 14.12.2019 04:31 Chewbacka2020
Astudent measures the mass of a beaker with an empty test tube in it, and finds that the mass is 156.852 grams. they then add approximately 15 ml of pure cyclohexane to the test tube and reweigh the beaker and test tube with cyclohexane in it. the beaker, test tube and cyclohexane weighs 171.206 grams. then they measure the freezing point of the pure cyclohexane and find that it is 6.60 degree c. the student then adds 0.300 grams of an unknown hydrocarbon to the cyclohexane in the test tube and stirs it until it has dissolved. finally, the freezing point of the cyclohexane and hydrocarbon solution was measured and found to be 1.25 degree c. the freezing point depression constant for cyclohexane is 20.2 degree c/m.
1. what is the change in temperature of the freezing point? delta t of freezing point:
2. what is the molality of the unknown hydrocarbon in the solution? molality of hydrocarbon:
3. what is the mass of cyclohexane in the test tube in kilograms? mass of cyclohexane:
4. how many moles of the unknown hydrocarbon are present in the solution? moles of hydrocarbon:
5. what is the molar mass of the unknown hydrocarbon? molar mass of hydrocarbon:

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

You know the right answer?
Astudent measures the mass of a beaker with an empty test tube in it, and finds that the mass is 156...
Questions


Mathematics, 29.06.2021 19:50


World Languages, 29.06.2021 19:50



Mathematics, 29.06.2021 19:50



Mathematics, 29.06.2021 19:50

Mathematics, 29.06.2021 20:00

Engineering, 29.06.2021 20:00


Physics, 29.06.2021 20:00



Mathematics, 29.06.2021 20:00

Mathematics, 29.06.2021 20:00


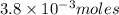
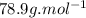 .
.