
Chemistry, 14.12.2019 07:31 andrejr0330jr
Nitrogen and oxygen can react to form various compounds. two experiments showed that one compound is formed when 3.62 g of nitrogen and 2.07 g of oxygen react completely, while another compound is formed when 1.82 g of nitrogen reacts completely with 4.13 g of oxygen. which of the following are most likely the molecular formulas for the nitrogen oxides obtained in these experiments? (1) no, n2o (2) no, no2 (3) n2o, n2o5 (4) no, n2o4 (5) n2o, n2o4

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1


Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
Nitrogen and oxygen can react to form various compounds. two experiments showed that one compound is...
Questions



Biology, 29.01.2020 15:51



English, 29.01.2020 15:51


Computers and Technology, 29.01.2020 15:51


Social Studies, 29.01.2020 15:51

Mathematics, 29.01.2020 15:51



Mathematics, 29.01.2020 15:51

Mathematics, 29.01.2020 15:51


Mathematics, 29.01.2020 15:52


Chemistry, 29.01.2020 15:52

Mathematics, 29.01.2020 15:52

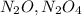
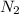 .
.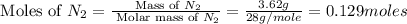
 .
.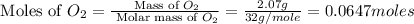
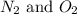 .
.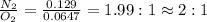
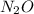 .
.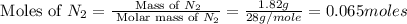
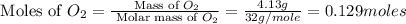
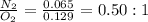
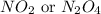 .
.

