
Chemistry, 14.12.2019 07:31 live4dramaoy0yf9
Predicting nuclear stability is important when determining whether or not a nuclear reaction can take place spontaneously. use the following set of guidelines to predict the nuclear stability of the elements listed below.
1. stable nuclei have a high neutron-to-proton ratio, greater than 1.25 beyond atomic number 40 and continuing to rise at higher atomic numbers.
2. the majority of stable nuclei have even numbers of neutrons and protons. odd-even and even-odd combinations can be stable but stability is less likely.
3. nuclei that have the magic numbers of 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82 or 126 neutrons are more stable than nuclei that do not contain these numbers.
4. nuclei that have atomic numbers greater than 83 are unstable. nuclei are considered stable if one or more criteria are met.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

You know the right answer?
Predicting nuclear stability is important when determining whether or not a nuclear reaction can tak...
Questions



Mathematics, 12.01.2020 05:31


Biology, 12.01.2020 05:31

Mathematics, 12.01.2020 05:31


Mathematics, 12.01.2020 05:31

Social Studies, 12.01.2020 05:31

History, 12.01.2020 05:31


Mathematics, 12.01.2020 05:31



English, 12.01.2020 05:31


Mathematics, 12.01.2020 05:31


Spanish, 12.01.2020 05:31

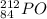
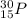
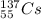
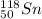
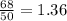 .It is more than 1.25.
.It is more than 1.25.

