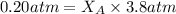Chemistry, 16.12.2019 17:31 kevinkingpin
Helium is mixed with oxygen gas for deep-sea divers. calculate the percent by volume of oxygen gas in the mixture if the diver has to submerge to a depth where the total pressure is 3.8 atm. the partial pressure of oxygen is maintained at 0.20 atm at this depth.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
Helium is mixed with oxygen gas for deep-sea divers. calculate the percent by volume of oxygen gas i...
Questions

Physics, 21.09.2019 08:10

Biology, 21.09.2019 08:10



History, 21.09.2019 08:10

Mathematics, 21.09.2019 08:10

Biology, 21.09.2019 08:10


History, 21.09.2019 08:10

Mathematics, 21.09.2019 08:10

History, 21.09.2019 08:10



Computers and Technology, 21.09.2019 08:10

Biology, 21.09.2019 08:10

Health, 21.09.2019 08:10



Advanced Placement (AP), 21.09.2019 08:10


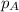 = total partial pressure of solution = 3.8 atm
= total partial pressure of solution = 3.8 atm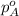 = partial pressure of oxygen = 0.20 atm
= partial pressure of oxygen = 0.20 atm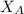 = mole fraction of oxygen = ?
= mole fraction of oxygen = ?