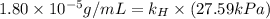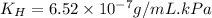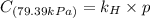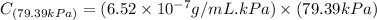Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

You know the right answer?
Exposing a 250 ml sample of water at 20.∘c to an atmosphere containing a gaseous solute at 27.59 kpa...
Questions


Physics, 06.04.2021 21:00


Business, 06.04.2021 21:00



Mathematics, 06.04.2021 21:00

Computers and Technology, 06.04.2021 21:00

Mathematics, 06.04.2021 21:00

Mathematics, 06.04.2021 21:00




Mathematics, 06.04.2021 21:00

Mathematics, 06.04.2021 21:00



Mathematics, 06.04.2021 21:00


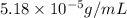
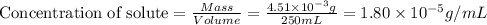
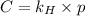
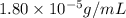
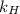 = Henry's law constant = ?
= Henry's law constant = ?