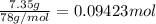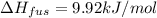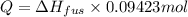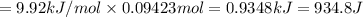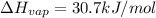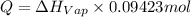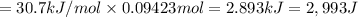Calculate the heat required to melt 7.35 g of benzene at its normal melting point. heat of fusion (benzene) = 9.92 kj/mol heat = kj
calculate the heat required to vaporize 7.35 g of benzene at its normal boiling point. heat of vaporization (benzene) = 30.7 kj/mol heat = kj

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Calculate the heat required to melt 7.35 g of benzene at its normal melting point. heat of fusion (b...
Questions



Mathematics, 21.12.2019 11:31








Biology, 21.12.2019 11:31


Computers and Technology, 21.12.2019 11:31



English, 21.12.2019 11:31

Mathematics, 21.12.2019 11:31

Mathematics, 21.12.2019 11:31


Mathematics, 21.12.2019 11:31