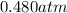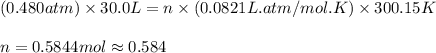Chemistry, 16.12.2019 18:31 Thejollyhellhound20
Carbon dioxide gas is collected at 27.0(degrees)c in an evacuated flask with a measured volume of 30.0l. when all the gas has been collected, the pressure in the flask is measured to be 0.480atm.
calculate the mass and number of moles of carbon dioxide gas that were collected. round your answer to 3 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1


Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

You know the right answer?
Carbon dioxide gas is collected at 27.0(degrees)c in an evacuated flask with a measured volume of 30...
Questions





Geography, 03.03.2020 19:44


Mathematics, 03.03.2020 19:44

Mathematics, 03.03.2020 19:44




Mathematics, 03.03.2020 19:44