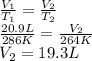An arctic weather balloon is filled with 20.9l of helium gas inside a prep shed. the temperature inside the shed is 13 degree c. the balloon is then taken outside, where the temperature is -9. degree c. calculate the new volume of the balloon. you may assume the pressure on the balloon stays constant at exactly 1atm. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 09:30
Northern was a learned a fairly cold climate caused by see one from the atlantic ocean, but se was real and tends to be much warmer, sonia look good causes difference. a.cool wins cannot blow across a leg into the south mountains, b.prevent cold air from blowing over into south sea,c.the south is at much higher elevation, so it is closer to the sun, d.suppose early and has a sperience a drastic climate change in the past few years.
Answers: 1
You know the right answer?
An arctic weather balloon is filled with 20.9l of helium gas inside a prep shed. the temperature ins...
Questions


Health, 01.12.2021 02:50

Mathematics, 01.12.2021 02:50

Biology, 01.12.2021 02:50


History, 01.12.2021 02:50


Social Studies, 01.12.2021 02:50

English, 01.12.2021 02:50

Mathematics, 01.12.2021 02:50


Mathematics, 01.12.2021 02:50



Biology, 01.12.2021 02:50


Mathematics, 01.12.2021 02:50

Biology, 01.12.2021 02:50


Mathematics, 01.12.2021 02:50