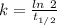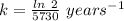Chemistry, 16.12.2019 18:31 bcifuentes
The previous part could be done without using the decay equation, because the ratio of original 14c to present 14c was an integer power of 1/2. most problems are not so simple. to solve more general carbon-dating problems, you must first find the value of the decay constant for 14c, so that you can easily use the decay equation. using the given half-life, 5730 years, find the value of the decay constant for 14c.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

You know the right answer?
The previous part could be done without using the decay equation, because the ratio of original 14c...
Questions

History, 27.04.2020 02:28







Mathematics, 27.04.2020 02:29

English, 27.04.2020 02:29

Physics, 27.04.2020 02:29

Mathematics, 27.04.2020 02:29

Social Studies, 27.04.2020 02:29

Biology, 27.04.2020 02:29

History, 27.04.2020 02:29

Biology, 27.04.2020 02:29

Mathematics, 27.04.2020 02:29

English, 27.04.2020 02:29

Mathematics, 27.04.2020 02:29

Mathematics, 27.04.2020 02:29

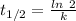
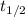 is the half life
is the half life