
Chemistry, 16.12.2019 21:31 cschellfamily
Each of the following sets of quantum numbers is supposed to specify an orbital. choose the one set of quantum numbers that does not contain an error. each of the following sets of quantum numbers is supposed to specify an orbital. choose the one set of quantum numbers that does not contain an error. n = 4, l = 4, ml =0 n = 4, l = 0, ml =+1 n = 5, l = 3, ml =-3 n = 3, l = 1, ml = +2 n = 3, l = 2, ml =-3

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
Each of the following sets of quantum numbers is supposed to specify an orbital. choose the one set...
Questions

English, 07.03.2021 16:10

History, 07.03.2021 16:10



Mathematics, 07.03.2021 16:10

English, 07.03.2021 16:20

Chemistry, 07.03.2021 16:20



Biology, 07.03.2021 16:20


Social Studies, 07.03.2021 16:20



Mathematics, 07.03.2021 16:20

Mathematics, 07.03.2021 16:20

Physics, 07.03.2021 16:20



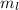 . The value of this quantum number ranges from
. The value of this quantum number ranges from 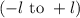 . When l = 2, the value of
. When l = 2, the value of 

