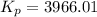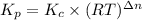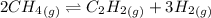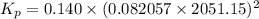Chemistry, 17.12.2019 00:31 jordantay208
For chemical reactions involving ideal gases, the equilibrium constant k can be expressed either in terms of the concentrations of the gases (in m) or as a function of the partial pressures of the gases (in atmospheres). in the latter case, the equilibrium constant is denoted as kp to distinguish it from the concentration-based equilibrium constant kc (sometimes referenced as just k).for the reaction 2ch4(g)⇌c2h2(g)+3h2(g) kc = 0.140 at 1778 ∘c . what is kp for the reaction at this temperature? express your answer numerically.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
For chemical reactions involving ideal gases, the equilibrium constant k can be expressed either in...
Questions


Arts, 26.02.2020 03:33





Mathematics, 26.02.2020 03:33


English, 26.02.2020 03:33





Mathematics, 26.02.2020 03:33

Mathematics, 26.02.2020 03:33




Computers and Technology, 26.02.2020 03:33