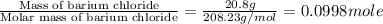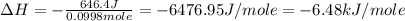Chemistry, 17.12.2019 00:31 raulhill98
An amount of solid barium chloride, 20.8 g, is dissolved in 100 g water in a coffee-cup calorimeter by the reaction: bacl2 (s) ba2+(aq) + 2cl−(aq) the water is originally at 25.0 °c and after the reaction the temperature of the solution is 26.6 °c. (cs = 4.04 j/(g°c) for the solution). what is the enthalpy change (δh) associated with the reaction as written?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

You know the right answer?
An amount of solid barium chloride, 20.8 g, is dissolved in 100 g water in a coffee-cup calorimeter...
Questions

Mathematics, 15.10.2020 21:01





Mathematics, 15.10.2020 21:01


Mathematics, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01


Mathematics, 15.10.2020 21:01

History, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01


Mathematics, 15.10.2020 21:01

Geography, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01


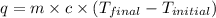
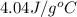
 = final temperature =
= final temperature = 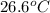
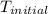 = initial temperature =
= initial temperature = 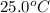
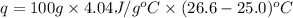
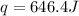
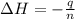
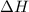 = enthalpy change = ?
= enthalpy change = ?