
Consider the following reaction at equilibrium. what effect will increasing the temperature have on the system? fe3o4(s) + co(g) ↔ 3 feo(s) + co2(g) δh°= +35.9 kjthe equilibrium constant will decrease. no effect will be observed. the reaction will shift to the right in the direction of products. the equilibrium constant will increase. the reaction will shift to the left in the direction of reactants

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
Consider the following reaction at equilibrium. what effect will increasing the temperature have on...
Questions





History, 07.10.2019 19:30


Mathematics, 07.10.2019 19:30










Mathematics, 07.10.2019 19:30



Computers and Technology, 07.10.2019 19:30

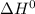 of the given reaction is positive and thus, on increasing temperature, reaction will go in forward direction.
of the given reaction is positive and thus, on increasing temperature, reaction will go in forward direction.

