Chemistry, 17.12.2019 01:31 shayla3613
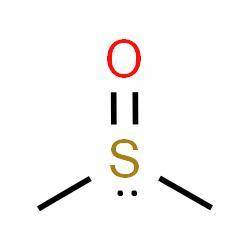
Dimethyl sulfoxide is an important polar aprotic solvent that can dissolve both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. because it penetrates the skin very readily, it is sometimes used as a vehicle for topical application of pharmaceuticals.
draw the structure of dimethyl sulfoxide. include any nonbonding electrons on sulfur, and minimize formal charges by allowing sulfur to expand its octet.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1


Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Dimethyl sulfoxide is an important polar aprotic solvent that can dissolve both polar and nonpolar c...
Questions






English, 09.12.2021 02:40

Mathematics, 09.12.2021 02:40

Social Studies, 09.12.2021 02:40



Mathematics, 09.12.2021 02:50

Mathematics, 09.12.2021 02:50

Biology, 09.12.2021 02:50

Advanced Placement (AP), 09.12.2021 02:50


History, 09.12.2021 02:50



Social Studies, 09.12.2021 02:50