

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
For each of the following pairs of substances, determine which has the larger molar entropy at 298 k...
Questions





Computers and Technology, 13.08.2019 00:20





Mathematics, 13.08.2019 00:20

Mathematics, 13.08.2019 00:20


Mathematics, 13.08.2019 00:20


Social Studies, 13.08.2019 00:20





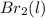 or
or 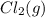 . Since, molar entropy of liquids is less than the molar entropy of gases.
. Since, molar entropy of liquids is less than the molar entropy of gases. 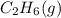 or
or 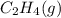 . As both the given species are gaseous in nature. So, more is the molar mass of specie more will be its molar entropy.
. As both the given species are gaseous in nature. So, more is the molar mass of specie more will be its molar entropy.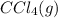 or
or 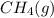 . Molar mass of
. Molar mass of 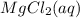 . Molar mass of NaCl 58.44 g/mol and molar mass of
. Molar mass of NaCl 58.44 g/mol and molar mass of 

