
Chemistry, 17.12.2019 02:31 titofigueroa777
Express the equilibrium constant for the following reaction. p4o10(s) ↔ p4(s) + 5 o2(g)k = [p4][o2]^5/[p4o10]k = [o2]^5k = [p4o10]/[p4][o2]^1/5k = [o2]^-5k = [p4o10]/[p4][o2]^5

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

You know the right answer?
Express the equilibrium constant for the following reaction. p4o10(s) ↔ p4(s) + 5 o2(g)k = [p4][o2]^...
Questions






Mathematics, 02.12.2020 21:10

Mathematics, 02.12.2020 21:10

Arts, 02.12.2020 21:10


History, 02.12.2020 21:10

Mathematics, 02.12.2020 21:10


Mathematics, 02.12.2020 21:10


Mathematics, 02.12.2020 21:20


History, 02.12.2020 21:20



![K=[O_2]^5](/tpl/images/0421/7050/87eee.png)
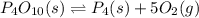
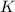 will be,
will be,

