
Chemistry, 17.12.2019 02:31 qmsadler01
The reaction 2nobr (g) → 2 no (g) + br2 (g) is a second-order reaction with a rate constant of 0.80 m-1s-1 at 11 °c. if the initial concentration of nobr is 0.0440 m, the concentration of nobr after 6.0 seconds is

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
The reaction 2nobr (g) → 2 no (g) + br2 (g) is a second-order reaction with a rate constant of 0.80...
Questions



Biology, 23.01.2021 06:50


English, 23.01.2021 06:50


English, 23.01.2021 06:50




Mathematics, 23.01.2021 06:50

Mathematics, 23.01.2021 06:50

Mathematics, 23.01.2021 06:50


Mathematics, 23.01.2021 06:50



History, 23.01.2021 06:50

Mathematics, 23.01.2021 06:50

![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0421/6919/ccade.png)
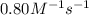
![[A_t]](/tpl/images/0421/6919/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0421/6919/dc622.png) = initial concentration = 0.0440 M
= initial concentration = 0.0440 M![0.80\times 6.0=\frac{1}{[A_t]}-\frac{1}{0.0440}](/tpl/images/0421/6919/0ff8f.png)
![[A_t]=0.036M](/tpl/images/0421/6919/f7d60.png)


