

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 07:00
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2
You know the right answer?
Express the equilibrium constant for the following reaction.2 k(s) + 2 h2o(l) ↔ 2 koh(aq) + h2(g)k =...
Questions





Computers and Technology, 10.11.2020 17:00







Mathematics, 10.11.2020 17:00



Chemistry, 10.11.2020 17:00

Mathematics, 10.11.2020 17:00




![K=[KOH]^2[H_2]](/tpl/images/0421/6907/b9417.png)
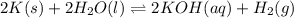
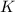 will be,
will be,

