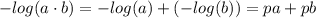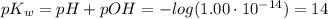Chemistry, 17.12.2019 02:31 Jasmineemarieee
The ph of a solution is the negative logarithm of the molar concentration of hydronium ion, that is, ph=−log[h3o+] in neutral solutions at 25 ∘c, [h3o+]=10−7 m and ph=7. as [h3o+] increases, ph decreases, so acidic solutions have a ph of less than 7. basic solutions have a ph greater than 7. the hydroxide and hydronium ion concentrations are related by the the ion-product constant of water, kw , as follows: kw=1.0×10−14=[h3o+][oh−] in the same way as the ph, we can define the poh as poh=−log[oh−]. it follows from the kw expression that ph+poh=14.

Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:02
Brainliesttt asap!me : ) who can read an article and answer some questions about it? ? only answer if you can.. : )
Answers: 2


Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
The ph of a solution is the negative logarithm of the molar concentration of hydronium ion, that is,...
Questions

Physics, 07.10.2020 07:01




Mathematics, 07.10.2020 07:01

Social Studies, 07.10.2020 07:01



Mathematics, 07.10.2020 07:01

Biology, 07.10.2020 07:01




Mathematics, 07.10.2020 07:01



Mathematics, 07.10.2020 07:01


Mathematics, 07.10.2020 07:01

Mathematics, 07.10.2020 07:01

![pH = -log[H^+]](/tpl/images/0421/6601/7d119.png)
![pH = -log[H^+], pK_a = -log(K_a)](/tpl/images/0421/6601/29e90.png) etc.
etc.![[H_3O^+] = [OH^-] = 1.00\cdot 10^{-7} M](/tpl/images/0421/6601/e5313.png)
![pH = -log[H_3O^+] = -log(1.00\cdot 10^{-7}) = 7.00](/tpl/images/0421/6601/b738b.png)
![[H_3O^+] =2.00\cdot 10^{-7} M](/tpl/images/0421/6601/25e55.png)
![pH = -log[H_3O^+] = -log(2.00\cdot 10^{-7}) = 6.70](/tpl/images/0421/6601/deab9.png)
![K_w=[H_3O^+][OH^-]](/tpl/images/0421/6601/16faa.png)
![K_w=[H_3O^+][OH^-]=1.00\cdot10^{-14}](/tpl/images/0421/6601/58549.png)
![-log(K_w)=-log([H_3O^+][OH^-])](/tpl/images/0421/6601/4aed2.png)