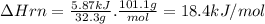Potassium nitrate, kno3, has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 32.3 g of kno3 is dissolved in 243 g of water at 23.00 °c. kno3(s)+h2o(aq) > koh(aq)+hno3(aq)the temperature of the resulting solution decreases to 17.90 °c. assume the resulting solution has the same specific heat as water, 4.184 j/(g·°c), and that there is negligible heat loss to the surroundings.1. how much heat was released by the solution? 2. what is the enthalpy of the reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
Potassium nitrate, kno3, has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 32.3 g...
Questions

Mathematics, 17.11.2020 18:30

Mathematics, 17.11.2020 18:30

History, 17.11.2020 18:30

History, 17.11.2020 18:30

English, 17.11.2020 18:30

Mathematics, 17.11.2020 18:30

Mathematics, 17.11.2020 18:30



Mathematics, 17.11.2020 18:30




Mathematics, 17.11.2020 18:30

Mathematics, 17.11.2020 18:30



English, 17.11.2020 18:30


History, 17.11.2020 18:30