Chemistry, 17.12.2019 03:31 20warriorsoul14
For which of the following processes will \deltaδs be negative? pbcl2(s) = pb2+(aq) + 2 cl-(aq)mgo(s) + co2(g) = mgco3(s)co2(aq) = co2(g)c5h12(l) + 8 o2(g) = 5 co2(g) + 6 h2o(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2


Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2
You know the right answer?
For which of the following processes will \deltaδs be negative? pbcl2(s) = pb2+(aq) + 2 cl-(aq)mgo(s...
Questions

Computers and Technology, 14.02.2020 19:42


English, 14.02.2020 19:43



Social Studies, 14.02.2020 19:44





Mathematics, 14.02.2020 19:45

English, 14.02.2020 19:45

Mathematics, 14.02.2020 19:45







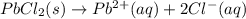 :
: 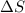 = +ve
= +ve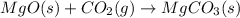 :
: 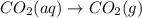 :
: 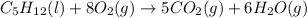 :
: