Which of the following gases would have the greatest kinetic energy at 300 k?
a. n2
b....


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Questions

Mathematics, 20.10.2019 15:30


Mathematics, 20.10.2019 15:30





English, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

Business, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30


English, 20.10.2019 15:30



Biology, 20.10.2019 15:30

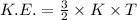
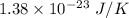
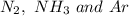 will posses same value of kinetic energy.
will posses same value of kinetic energy.


