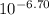Chemistry, 17.12.2019 04:31 Trishamw04
Astudent was given a 50.0 ml of 0.10 m solution of an unknown diprotic acid, h2a, which was titrated with 0.10 m naoh. after a total of 25.0 ml of naoh was added, the resulting solution has a ph of 6.70.
after a total of 50.0 ml of naoh was added, the ph of the solution was 8.00.
determine the values of ka1 and ka2 for the diprotic acid.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1
You know the right answer?
Astudent was given a 50.0 ml of 0.10 m solution of an unknown diprotic acid, h2a, which was titrated...
Questions







History, 19.03.2020 00:19


Mathematics, 19.03.2020 00:19


English, 19.03.2020 00:19





History, 19.03.2020 00:19

Social Studies, 19.03.2020 00:19